Lidocaine Hydrochloride Injection 100mg/5mL × 5 | For Export Only
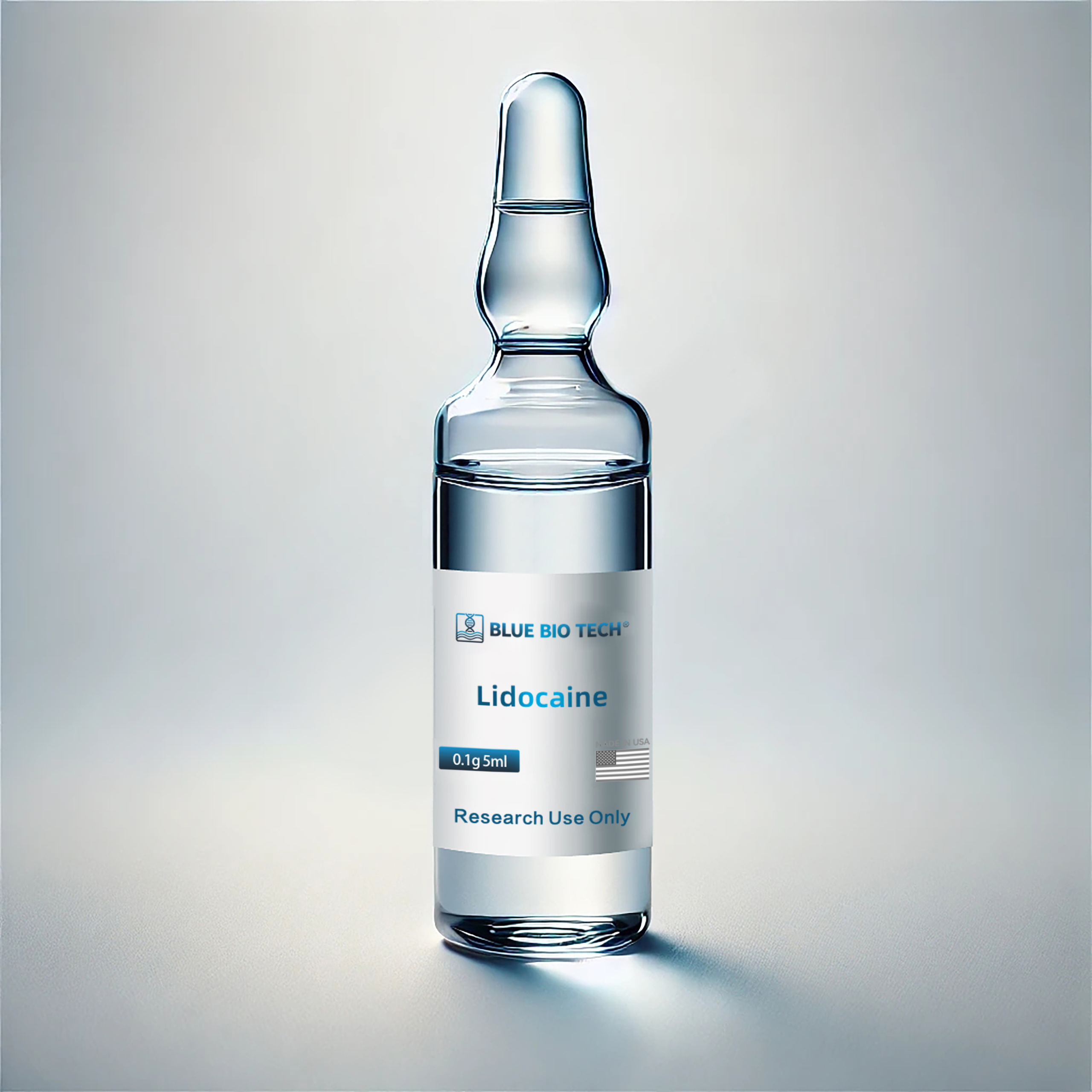
Lidocaine Hydrochloride Injection (Rx) – 100mg/5mL × 5 Ampoules
Sterile Local Anesthetic for Clinical & Aesthetic Use | BlueBiotech® Global Supply | For Export Only
Lidocaine Hydrochloride Injection 100mg/5mL by BlueBiotech® provides rapid-onset, reversible local anesthesia for aesthetic procedures, minor surgeries, and infiltration use. This sterile injectable is manufactured in Asia under strict quality protocols and clearly labeled “For Export Only,” not approved for sale or use in the United States domestic market.
| Field Name | Example Value |
|---|---|
| Product Name | Lidocaine Hydrochloride Injection (Rx) – 100mg/5mL × 5 Ampoules | Sterile Local Anesthetic | For Export Only |
| Product Code | LID-HK-01-335823 |
| Product Type | Injectable / Local Anesthetic / Clinical Adjuvant |
| Category | Skin Health / Aesthetic Support / Surgical Preparation |
| Manufacturer | BlueBiotech® Global Supply |
| Appearance | Clear sterile solution in sealed ampoules |
| Dosage / Units | 100mg lidocaine hydrochloride per 5mL ampoule |
| Purity | ≥99% pharmaceutical-grade Lidocaine HCl; preservative-free, sterile |
| Quantity Options | Box of 5 ampoules |
| Storage | Store at 2–25°C; protect from light and excessive heat |
| Shelf Life | 24 months unopened |
| Packaging | Medical export packaging with lot number, bilingual insert (English/Chinese), and “For Export Only” compliance label |
| Manufacturer Origin | Non-U.S. Origin – Manufactured in Asia (e.g., China, Taiwan, South Korea, or Japan) |
| Use Classification | For Export Only – For institutional/professional use only; not for use or sale in the United States |
| Tags | lidocaine hydrochloride, injectable anesthetic, local anesthesia, BLUEBIOTECH, clinical use only, for export only |
⚠️This product is labeled for Research Use Only (RUO). Not for human or veterinary therapeutic use.
See RUO Declaration for more info.
Lidocaine Hydrochloride Injection 100mg/5mL × 5 Ampoules | Local Anesthetic for Clinical Use | For Export Only
Product Overview
Lidocaine Hydrochloride Injection is a fast-acting local anesthetic used for regional anesthesia, nerve blocks, and minor surgical procedures. This formulation contains 100mg of Lidocaine per 5mL ampoule, offering effective pain relief and temporary loss of sensation in the targeted area.
Distributed by BlueBiotech® via its global pharmaceutical supply chain, this product is manufactured under GMP standards and is clearly labeled For Export Only. It is intended for use by licensed medical professionals outside the United States.
🔬 Learn more: PubMed – Lidocaine Hydrochloride Injection
Key Features & Benefits
-
✅ 100mg/5mL per Ampoule – Clinically relevant dose for regional anesthesia and infiltration
-
✅ Rapid Onset (1–3 minutes) – Intermediate duration of action (30–90 minutes)
-
✅ Widely Used in Medical & Aesthetic Procedures
-
✅ Clear, Colorless Sterile Solution – Suitable for IM, SC, or local injection
-
✅ Export Packaging – 5 Ampoules per Box
Product Specifications
-
Product Name: Lidocaine Hydrochloride Injection
-
Strength: 100mg per 5mL
-
Active Ingredient: Lidocaine Hydrochloride
-
Form: Sterile aqueous injectable solution
-
Packaging: 5 ampoules per box
-
Route of Administration: Subcutaneous (SC), intramuscular (IM), or per procedural requirement
-
Origin: International manufacturing partner; distributed by BlueBiotech®
-
Storage Conditions: 15°C to 25°C; protect from light and freezing
-
Shelf Life: 24 months
Clinical Applications
-
Local anesthesia for dental, dermatological, or minor surgical procedures
-
Peripheral nerve block or field block anesthesia
-
Pain management in cosmetic injectable procedures
-
Adjuvant in mesotherapy, thread lifting, or laser-based skin treatments
-
Occasionally used under supervision in cardiac arrhythmia management
How It Works
Lidocaine Hydrochloride works by reversibly blocking sodium channels in nerve membranes, preventing the initiation and conduction of nerve impulses. This mechanism leads to temporary numbness, allowing for pain-free procedures. It has a rapid onset of action, making it ideal for short to medium-duration interventions across medical and aesthetic disciplines.
⚠️ Compliance & Export Statement
For Export Only – This product is not approved for sale, use, or distribution within the United States.
It is intended for licensed medical practitioners in international markets where the use of injectable lidocaine is legally permitted.
All importers, clinics, and end users are responsible for ensuring compliance with their local health regulations.
Tags / Keywords
Lidocaine Hydrochloride Injection 100mg, Injectable Local Anesthetic, Nerve Block Injection, Lidocaine 5mL Ampoule, For Export Only Lidocaine, BlueBiotech Clinical Lidocaine, Cosmetic Procedure Anesthesia, GMP Injectable Anesthetic, Lidocaine for Minor Surgery, Injectable Pain Control
You might also like
Similar Products
Corporate Advantages

High-Purity Standards
Tested for purity and identity. COAs available
for all RUO compounds

Buyer Protection
Protected and discrete fulfillment
available upon request

Research-Grade Support
Dedicated service for labs, clinics, and
compounders handling RUO items.

Lab-Grade Quality Assurance
All compounds are tested for-identity and purity, COAs and supporting documentation are available upon request.

Strict RUO Compliance
All products are labeled for Researd Use Only (RUO), Not intended for human or veterinary therapeutic or diagnostic purposes.
Lab-Grade Quality Assurance

Custom branding.
white-label optionons and formulation assistancee available for eligible research institutions and partners.
Custom R&D & APl Synthesis

We support original
pharmaceutica solutions.custom research projects, and NDC-grade materials-meeting speifica.tions for qualified partners.

Serving Professionals Across the U.S.& Mexico
Trusted by MedSpas, wellness clinics and licensed compounders under licensed compounding relationships.
Secure & Discreet Fulfillment
Cold-chain shipping : private, plain.wrap packaging, and tracking for sensitive RUO materials.

⚠️ All compounds are provided strictly for Research Use Only (RUO). BLUEBIOTECH does not endorse or support clinical, therapeutic, or diagnostic use of its products unless under licensed compounder supervision.






![Meditoxin - Botulinum Toxin Type A [South Korea]](https://www.bluebiotech.health/wp-content/uploads/2024/12/Meditoxin-Type-A-100ui-1-600x800.jpg)