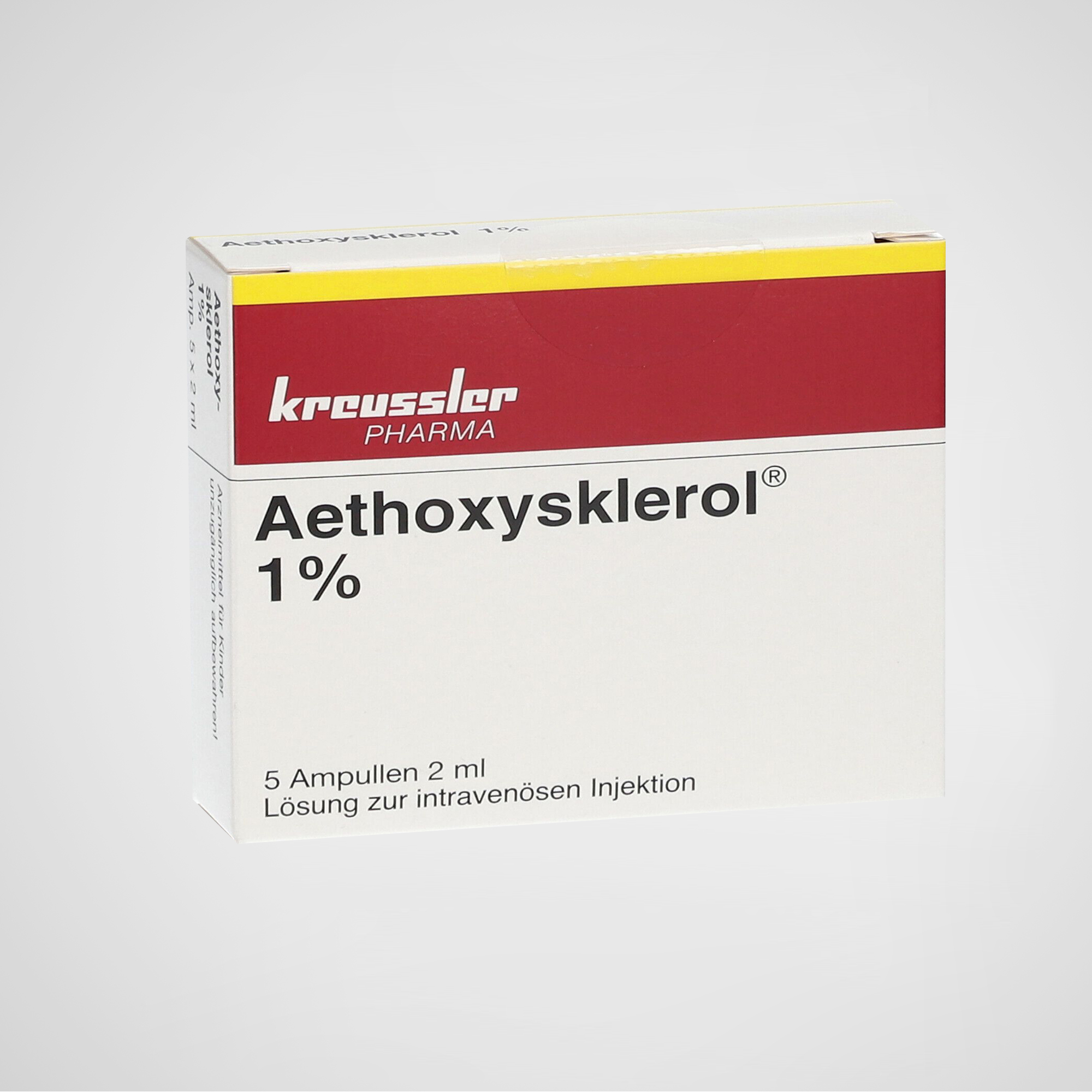
Aethoxysklerol® 1% – Polidocanol Injection
| Field Name | Example Value |
|---|---|
| Product Name | Aethoxysklerol 1% – Polidocanol Injection | European Origin | Sclerotherapy Agent |
| Product Code | AET-EU-1 |
| Product Type | Branded Pharmaceutical / Sclerosing Agent |
| Category | Vascular / Dermatology / Injectable Solutions |
| Manufacturer | Kreussler Pharma (Germany) |
| Appearance | Clear, colorless sterile solution in ampoule or vial |
| Dosage / Units | 1% Polidocanol (10mg/mL), 2mL or 5mL per vial/ampoule |
| Purity | GMP-grade injectable solution |
| Quantity Options | Box of 5 ampoules / Box of 10 ampoules |
| Storage | Store below 25°C; protect from light; do not freeze |
| Shelf Life | 36 months unopened |
| Packaging | Original European packaging with multilingual leaflet and batch serialization |
| Manufacturer Origin | Germany |
| Use Classification | Export Only – For clinical research, institutional, or licensed medical use per destination regulations |
| Tags | aethoxysklerol, polidocanol, sclerotherapy, spider veins, varicose, injectable foam, Kreussler, BLUEBIOTECH |
⚠️This product is labeled for Research Use Only (RUO). Not for human or veterinary therapeutic use.
See RUO Declaration for more info.
Aethoxysklerol® 1% – Polidocanol Injection | European-Made Sclerosing Agent for Global Medical Supply
Product Overview
Aethoxysklerol® (polidocanol injection) is a high-purity sclerosing agent widely used for the treatment of varicose veins, spider veins, and hemorrhoids. It is manufactured in Germany by Kreussler Pharma, a globally recognized pharmaceutical company specializing in vascular and dermatological therapeutics.
This version of Aethoxysklerol® contains 1% polidocanol (10 mg/mL), formulated for intravenous or intralesional use by trained healthcare professionals. It is part of the manufacturer’s global distribution supply, and is identical in composition to versions approved by the EMA and other international health authorities. Packaging and labeling may vary based on regional regulatory requirements.
Key Features & Advantages
-
✅ European-Made by Kreussler Pharma (Germany)
Trusted manufacturer with decades of experience in sclerotherapy solutions. -
✅ Standardized 1% Polidocanol Formulation
Ideal for treating telangiectasias and small varicose veins (CEAP C1–C2 classification). -
✅ Clinically Proven Sclerosing Effect
Causes endothelial damage and vein closure with minimal patient discomfort. -
✅ Favorable Safety Profile
Low risk of hyperpigmentation, matting, or allergic reaction when administered properly. -
✅ Global Compatibility
Widely used in Europe, Asia, and South America; suitable for export and institutional purchase.
Product Specifications
-
Product Name: Aethoxysklerol® 1%
-
Active Ingredient: Polidocanol (lauromacrogol 400)
-
Concentration: 10 mg/mL (1%)
-
Form: Injectable solution
-
Presentation: 2 mL or 5 mL ampoules / vials (depending on market)
-
Route of Administration: Intravenous (IV) / Intralesional
-
Manufacturer: Kreussler Pharma (Germany)
-
Packaging: Global/Export version with destination-specific labeling
-
Storage: Store below 25°C; protect from light
-
Shelf Life: Typically 36 months from manufacture
-
Classification: Original brand; not generic
Mechanism of Action
Polidocanol acts as a surface-active agent that induces controlled endothelial damage upon direct contact with the vein wall. This leads to platelet aggregation, localized inflammation, and eventual fibrotic closure of the treated vessel.
Aethoxysklerol® 1% is best suited for:
-
Reticular veins
-
Spider veins (telangiectasias)
-
Early-stage varicose veins
-
Hemorrhoidal therapy (off-label in some markets)
The 1% concentration provides an effective balance between therapeutic action and patient tolerability, especially in cosmetic sclerotherapy settings.
Reference: PubMed Polidocanol Clinical Review
Compliance Statement
This product is manufactured in Germany by Kreussler Pharma and supplied globally under international pharmaceutical trade standards. The formulation is identical to the EU-approved Aethoxysklerol®. Only the packaging and labeling may vary based on the destination country’s regulatory requirements. It is intended for administration by licensed medical professionals only. All importers and users are responsible for ensuring compliance with local healthcare regulations and licensing.
Tags / Keywords
Aethoxysklerol 1%, Polidocanol Injection, Sclerotherapy Agent, Varicose Vein Treatment, German-Made Sclerosant, Kreussler Pharma, Spider Vein Removal, Hemorrhoid Sclerotherapy, Cosmetic Phlebology, Vascular Injection Therapy
You might also like
Similar Products
Corporate Advantages

High-Purity Standards
Tested for purity and identity. COAs available
for all RUO compounds

Buyer Protection
Protected and discrete fulfillment
available upon request

Research-Grade Support
Dedicated service for labs, clinics, and
compounders handling RUO items.

Lab-Grade Quality Assurance
All compounds are tested for-identity and purity, COAs and supporting documentation are available upon request.

Strict RUO Compliance
All products are labeled for Researd Use Only (RUO), Not intended for human or veterinary therapeutic or diagnostic purposes.
Lab-Grade Quality Assurance

Custom branding.
white-label optionons and formulation assistancee available for eligible research institutions and partners.
Custom R&D & APl Synthesis

We support original
pharmaceutica solutions.custom research projects, and NDC-grade materials-meeting speifica.tions for qualified partners.

Serving Professionals Across the U.S.& Mexico
Trusted by MedSpas, wellness clinics and licensed compounders under licensed compounding relationships.
Secure & Discreet Fulfillment
Cold-chain shipping : private, plain.wrap packaging, and tracking for sensitive RUO materials.

⚠️ All compounds are provided strictly for Research Use Only (RUO). BLUEBIOTECH does not endorse or support clinical, therapeutic, or diagnostic use of its products unless under licensed compounder supervision.