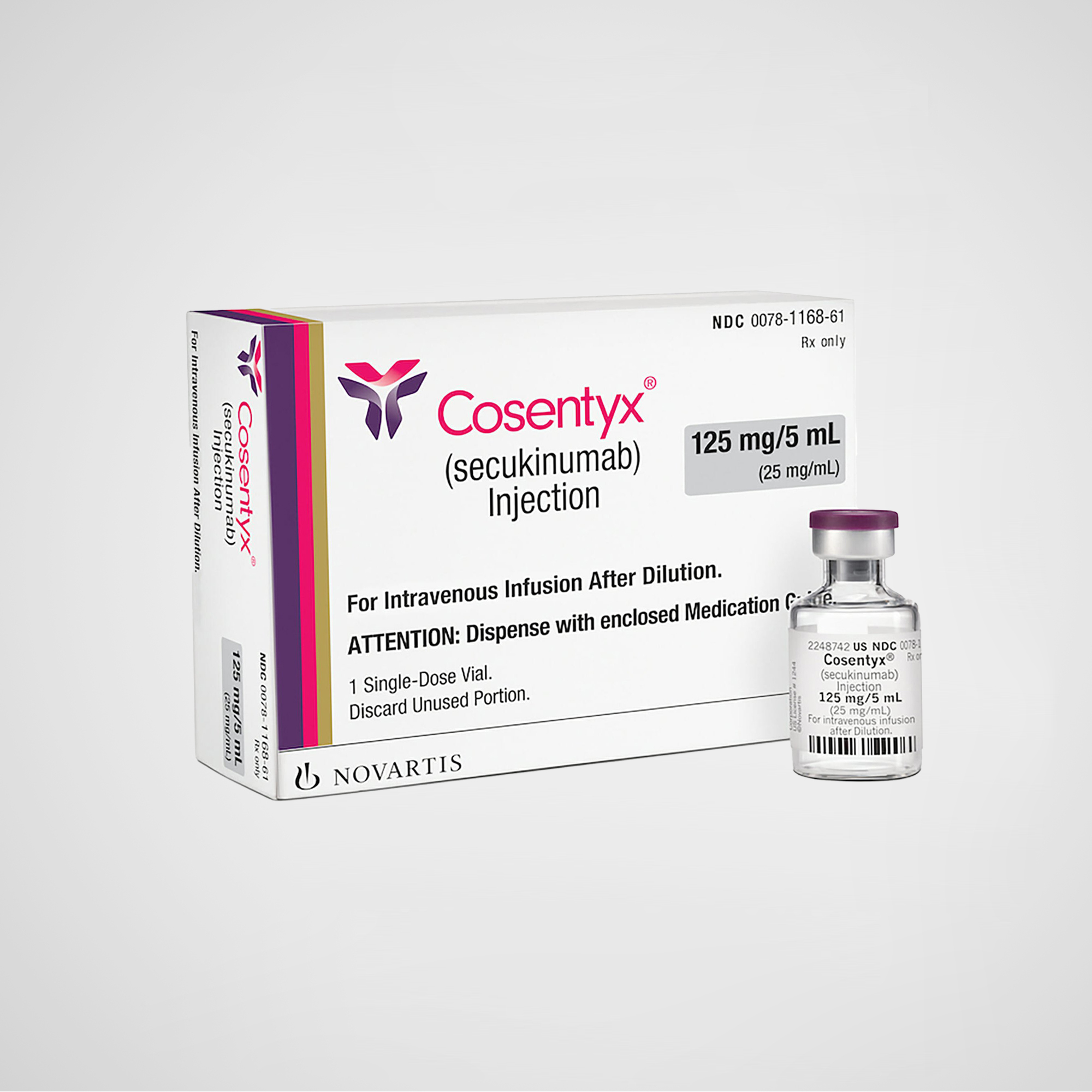
Cosentyx® – Secukinumab Injection
Cosentyx® (secukinumab) is a fully human monoclonal antibody that selectively binds to and neutralizes interleukin-17A (IL-17A). It is approved for the treatment of moderate-to-severe plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, and non-radiographic axial spondyloarthritis. This version is U.S.-manufactured and internationally packaged for compliant export.
| Field Name | Example Value |
|---|---|
| Product Name | Cosentyx® – Secukinumab Injection [U.S.-Manufactured |
| Product Code | COS-USA-150 |
| Product Type | Branded Biologic / Monoclonal Antibody |
| Category | Immunology / Monoclonal Antibodies / Psoriasis |
| Manufacturer | Novartis Pharmaceuticals Corporation (USA) |
| Appearance | Clear to slightly opalescent liquid in pre-filled syringe or autoinjector |
| Dosage / Units | 150mg/mL per syringe (or 2×150mg for 300mg dosing regimens) |
| Purity | Pharmaceutical grade (sterile, preservative-free, originator biologic) |
| Quantity Options | 1 syringe / 2 syringes / 6 syringes |
| Storage | Refrigerate at 2–8°C; do not freeze; protect from direct light |
| Shelf Life | 24 months unopened |
| Packaging | Global packaging with multilingual insert and serial-labeled export units (EU/FDA-compliant format) |
| Manufacturer Origin | United States |
| Use Classification | Export Only – For institutional, research, or regulatory evaluation use outside U.S. domestic market |
| Tags | cosentyx, secukinumab, IL-17A inhibitor, psoriasis, psoriatic arthritis, ankylosing spondylitis, BLUEBIOTECH |
⚠️This product is labeled for Research Use Only (RUO). Not for human or veterinary therapeutic use.
See RUO Declaration for more info.
Cosentyx® – Secukinumab Injection | U.S.-Manufactured Global Supply Monoclonal Antibody
Product Overview
Cosentyx® (secukinumab) is a fully human IgG1/κ monoclonal antibody that targets interleukin-17A (IL-17A), developed and manufactured by Novartis Pharmaceuticals Corporation in the United States. It is the world’s first approved IL-17A inhibitor, indicated for a broad range of immune-mediated inflammatory diseases including plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, and others.
The product offered here is part of the official global supply chain and is manufactured in the United States by the originator company. It is intended for international distribution under local regulatory requirements. While the packaging language and labeling may vary based on destination market regulations, the formulation, dosage form, device, and manufacturing quality are identical to the U.S.-marketed version.
Key Features & Advantages
-
✅ U.S.-Manufactured by Novartis Pharmaceuticals
Original biologic drug made under strict FDA and global cGMP standards. -
✅ Same Composition as U.S. Version
Identical formulation, concentration, and delivery system; only packaging language is adapted for local compliance. -
✅ First-in-Class IL-17A Blocker
Approved in over 100 countries; extensive real-world safety and efficacy data. -
✅ Multiple Indications
Includes moderate-to-severe plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, non-radiographic axial spondyloarthritis, and hidradenitis suppurativa (availability and indication vary by region). -
✅ Global Supply Ready
Suitable for licensed importers, hospitals, international health systems, and registered distributors.
Product Specifications
-
Product Name: Cosentyx® (secukinumab injection)
-
Active Ingredient: Secukinumab (recombinant human IgG1 monoclonal antibody)
-
Strength / Form: 150 mg/mL or 300 mg/2 mL prefilled syringe / autoinjector
-
Administration: Subcutaneous injection
-
Manufacturer: Novartis Pharmaceuticals Corporation (USA)
-
Packaging: International version with destination-specific labeling; drug content identical to U.S. formulation
-
Storage: 2°C–8°C refrigerated; do not freeze
-
Shelf Life: Typically 24 months from manufacture
-
Supply Category: Original brand biologic; not a biosimilar or generic
Mechanism of Action
Cosentyx® works by selectively binding and neutralizing interleukin-17A (IL-17A), a cytokine that plays a central role in chronic inflammation of the skin, joints, and spine. By blocking IL-17A, secukinumab inhibits inflammatory signaling and reduces the immune system’s abnormal activity.
Clinically, this results in:
-
Rapid clearance of psoriatic plaques
-
Reduced joint inflammation and long-term structural protection
-
Improved spinal mobility and pain control in axial spondyloarthritis
Reference: NIH: Mechanism of Secukinumab
Compliance Statement
This product is manufactured in the United States by Novartis Pharmaceuticals Corporation and is distributed as part of its international supply chain. It is identical in composition and manufacturing standard to the U.S.-market version, with only packaging and labeling adapted for regulatory compliance in export markets. This product is intended for lawful use by licensed importers, medical institutions, and distribution partners. All recipients are responsible for ensuring compliance with local pharmaceutical laws and regulations.
Tags / Keywords
Cosentyx International Version, Secukinumab Global Supply, IL-17A Monoclonal Antibody, Novartis Biologic, U.S.-Manufactured MAb, Autoimmune Biologic Therapy, Psoriasis Injection, Ankylosing Spondylitis Treatment, Global Distribution Biologics, Non-Biosimilar Secukinumab
You might also like
Similar Products
Corporate Advantages

High-Purity Standards
Tested for purity and identity. COAs available
for all RUO compounds

Buyer Protection
Protected and discrete fulfillment
available upon request

Research-Grade Support
Dedicated service for labs, clinics, and
compounders handling RUO items.

Lab-Grade Quality Assurance
All compounds are tested for-identity and purity, COAs and supporting documentation are available upon request.

Strict RUO Compliance
All products are labeled for Researd Use Only (RUO), Not intended for human or veterinary therapeutic or diagnostic purposes.
Lab-Grade Quality Assurance

Custom branding.
white-label optionons and formulation assistancee available for eligible research institutions and partners.
Custom R&D & APl Synthesis

We support original
pharmaceutica solutions.custom research projects, and NDC-grade materials-meeting speifica.tions for qualified partners.

Serving Professionals Across the U.S.& Mexico
Trusted by MedSpas, wellness clinics and licensed compounders under licensed compounding relationships.
Secure & Discreet Fulfillment
Cold-chain shipping : private, plain.wrap packaging, and tracking for sensitive RUO materials.

⚠️ All compounds are provided strictly for Research Use Only (RUO). BLUEBIOTECH does not endorse or support clinical, therapeutic, or diagnostic use of its products unless under licensed compounder supervision.







![Ozempic® Semaglutide Injection 4.0mg/3.0ml [China Edition]](https://www.bluebiotech.health/wp-content/uploads/2024/11/3-2-600x800.jpg)