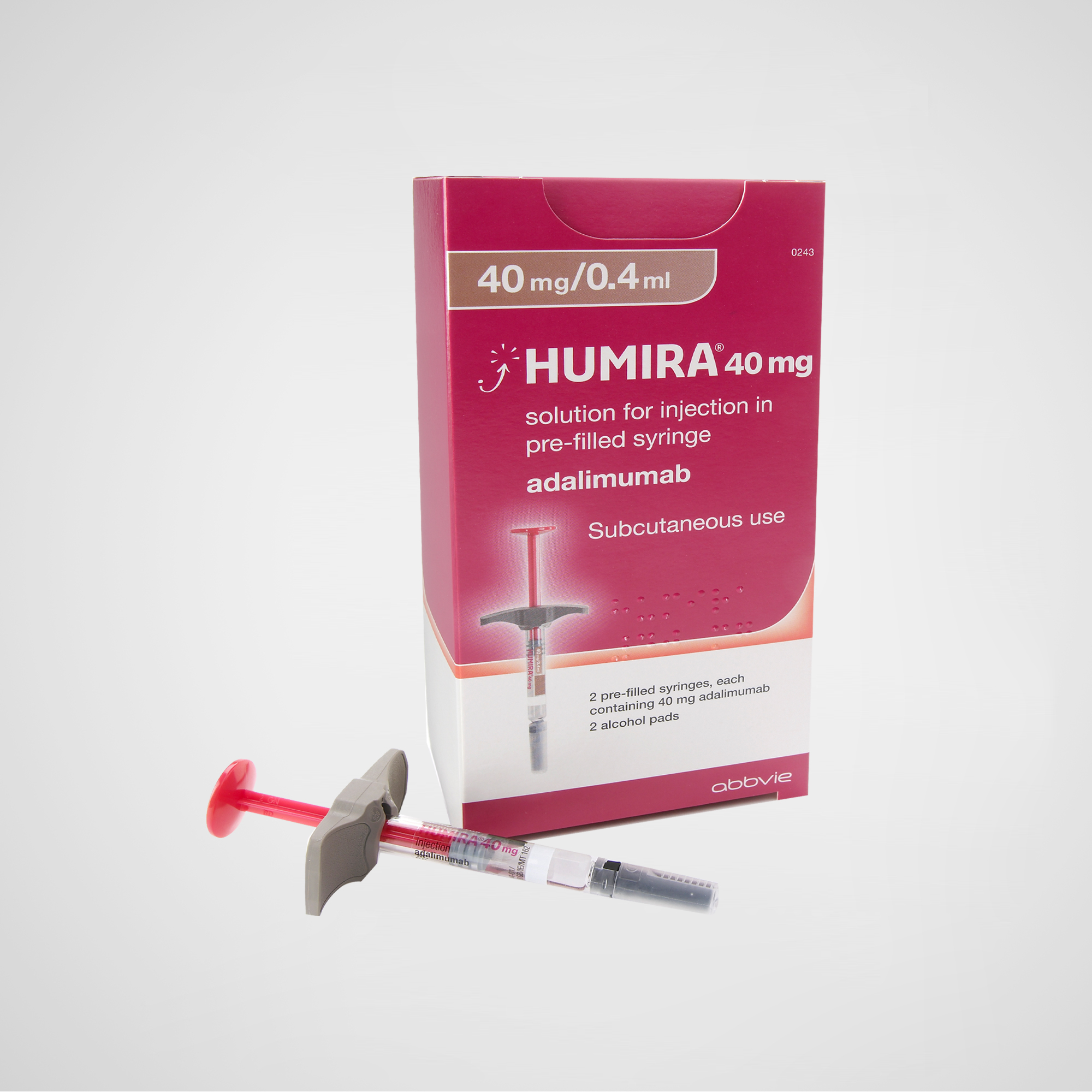
Humira® – Adalimumab Injection
Humira® (adalimumab) is a fully human monoclonal antibody that targets tumor necrosis factor alpha (TNF-α). It is indicated for a wide range of autoimmune diseases including rheumatoid arthritis, Crohn’s disease, ulcerative colitis, psoriasis, and ankylosing spondylitis. U.S.-manufactured, with global packaging suitable for compliant export.
| Field Name | Example Value |
|---|---|
| Product Name | Humira® – Adalimumab Injection | U.S.-Manufactured | TNF Inhibitor Biologic |
| Product Code | HUM-USA-40 |
| Product Type | Branded Biologic / TNF-α Inhibitor |
| Category | Autoimmune / Rheumatology / Dermatology / Gastroenterology |
| Manufacturer | AbbVie Inc. |
| Appearance | Clear, colorless to slightly yellow solution in pre-filled syringe or autoinjector |
| Dosage / Units | 40mg/0.8mL (standard adult dose) |
| Purity | Pharmaceutical grade (originator biologic; sterile, preservative-free) |
| Quantity Options | 1 syringe / 2 syringes / 6-syringe pack |
| Storage | Store refrigerated at 2–8°C; do not freeze; protect from light |
| Shelf Life | 24 months unopened |
| Packaging | Global-standard packaging with multilingual leaflet and serialization (FDA/EU compliant) |
| Manufacturer Origin | United States |
| Use Classification | Export Only – For institutional, research, or regulatory evaluation use outside the U.S. domestic market |
| Tags | humira, adalimumab, TNF inhibitor, autoimmune biologic, rheumatoid arthritis, AbbVie, original biologic, BLUEBIOTECH |
⚠️This product is labeled for Research Use Only (RUO). Not for human or veterinary therapeutic use.
See RUO Declaration for more info.
Humira® – Adalimumab Injection | U.S.-Manufactured Global Supply TNF-α Inhibitor
Product Overview
Humira® (adalimumab) is a fully human IgG1 monoclonal antibody that targets tumor necrosis factor-alpha (TNF-α) — a key mediator in chronic inflammatory and autoimmune disorders. Developed and manufactured by AbbVie Inc. (USA), Humira® is one of the most widely prescribed biologic therapies globally, with approvals in over 100 countries.
The version offered here is manufactured in the United States by the originator company and distributed through AbbVie’s global pharmaceutical supply chain. While packaging and labeling may vary by regulatory requirements in the destination market, the formulation, device system, and quality standards are identical to the U.S.-marketed product.
Key Features & Advantages
-
✅ U.S.-Manufactured Original Product
Produced by AbbVie Inc. under FDA and global GMP standards. -
✅ Global Standard Formulation
Only outer carton and leaflet are localized; drug content and autoinjector/syringe system remain identical. -
✅ One of the Most Prescribed Biologics Worldwide
Proven efficacy across a broad range of autoimmune and inflammatory diseases. -
✅ Approved in Over 100 Countries
Supported by 20+ years of safety and efficacy data in real-world and clinical settings. -
✅ Reliable Global Supply
Available to licensed importers, hospitals, and institutional buyers.
Product Specifications
-
Product Name: Humira® (adalimumab injection)
-
Active Ingredient: Adalimumab (recombinant human IgG1 monoclonal antibody)
-
Strength / Form: 40 mg/0.8 mL prefilled syringe or pen (standard); other strengths available depending on region
-
Administration: Subcutaneous injection
-
Manufacturer: AbbVie Inc. (USA)
-
Packaging: Destination-market specific labeling (e.g., Chinese, English, Arabic); identical formulation globally
-
Storage: 2°C–8°C; do not freeze
-
Shelf Life: 24 months from manufacture
-
Supply Type: Originator biologic; not a biosimilar
Mechanism of Action
Humira® binds specifically to TNF-α, neutralizing its biological activity by preventing interaction with its surface receptors (p55 and p75) on inflammatory cells. By inhibiting TNF-α, Humira® reduces systemic inflammation and halts the autoimmune-driven damage characteristic of multiple chronic conditions.
Clinical outcomes include:
-
Reduced joint swelling and structural damage in RA and PsA
-
Lowered disease activity in IBD and axial spondyloarthritis
-
Significant clearance of skin lesions in psoriasis
-
Long-term disease control across indications
More: NIH: Adalimumab Mechanism Overview
Indications (may vary by region)
-
Rheumatoid Arthritis (RA)
-
Psoriatic Arthritis (PsA)
-
Ankylosing Spondylitis (AS)
-
Crohn’s Disease (CD)
-
Ulcerative Colitis (UC)
-
Plaque Psoriasis (PP)
-
Hidradenitis Suppurativa (HS)
-
Juvenile Idiopathic Arthritis (JIA)
-
Uveitis (non-infectious)
Compliance Statement
This product is manufactured in the United States by AbbVie Inc. and is distributed internationally through the originator’s official global supply network. The formulation, strength, and delivery system are identical to the U.S.-approved product. Only packaging language and regulatory markings may differ by region. This product is not a biosimilar. Licensed importers and healthcare institutions are responsible for compliance with applicable local regulations.
Tags / Keywords
Humira, Adalimumab Injection, Original TNF-Alpha Inhibitor, AbbVie Biologic, U.S.-Manufactured Humira, Global Supply Biologics, Rheumatoid Arthritis Monoclonal Antibody, Crohn’s Disease Biologic, Psoriasis Therapy, Not a Biosimilar
You might also like
Similar Products
Corporate Advantages

High-Purity Standards
Tested for purity and identity. COAs available
for all RUO compounds

Buyer Protection
Protected and discrete fulfillment
available upon request

Research-Grade Support
Dedicated service for labs, clinics, and
compounders handling RUO items.

Lab-Grade Quality Assurance
All compounds are tested for-identity and purity, COAs and supporting documentation are available upon request.

Strict RUO Compliance
All products are labeled for Researd Use Only (RUO), Not intended for human or veterinary therapeutic or diagnostic purposes.
Lab-Grade Quality Assurance

Custom branding.
white-label optionons and formulation assistancee available for eligible research institutions and partners.
Custom R&D & APl Synthesis

We support original
pharmaceutica solutions.custom research projects, and NDC-grade materials-meeting speifica.tions for qualified partners.

Serving Professionals Across the U.S.& Mexico
Trusted by MedSpas, wellness clinics and licensed compounders under licensed compounding relationships.
Secure & Discreet Fulfillment
Cold-chain shipping : private, plain.wrap packaging, and tracking for sensitive RUO materials.

⚠️ All compounds are provided strictly for Research Use Only (RUO). BLUEBIOTECH does not endorse or support clinical, therapeutic, or diagnostic use of its products unless under licensed compounder supervision.






![Ozempic® Semaglutide Injection 4.0mg/3.0ml [China Edition]](https://www.bluebiotech.health/wp-content/uploads/2024/11/3-2-600x800.jpg)