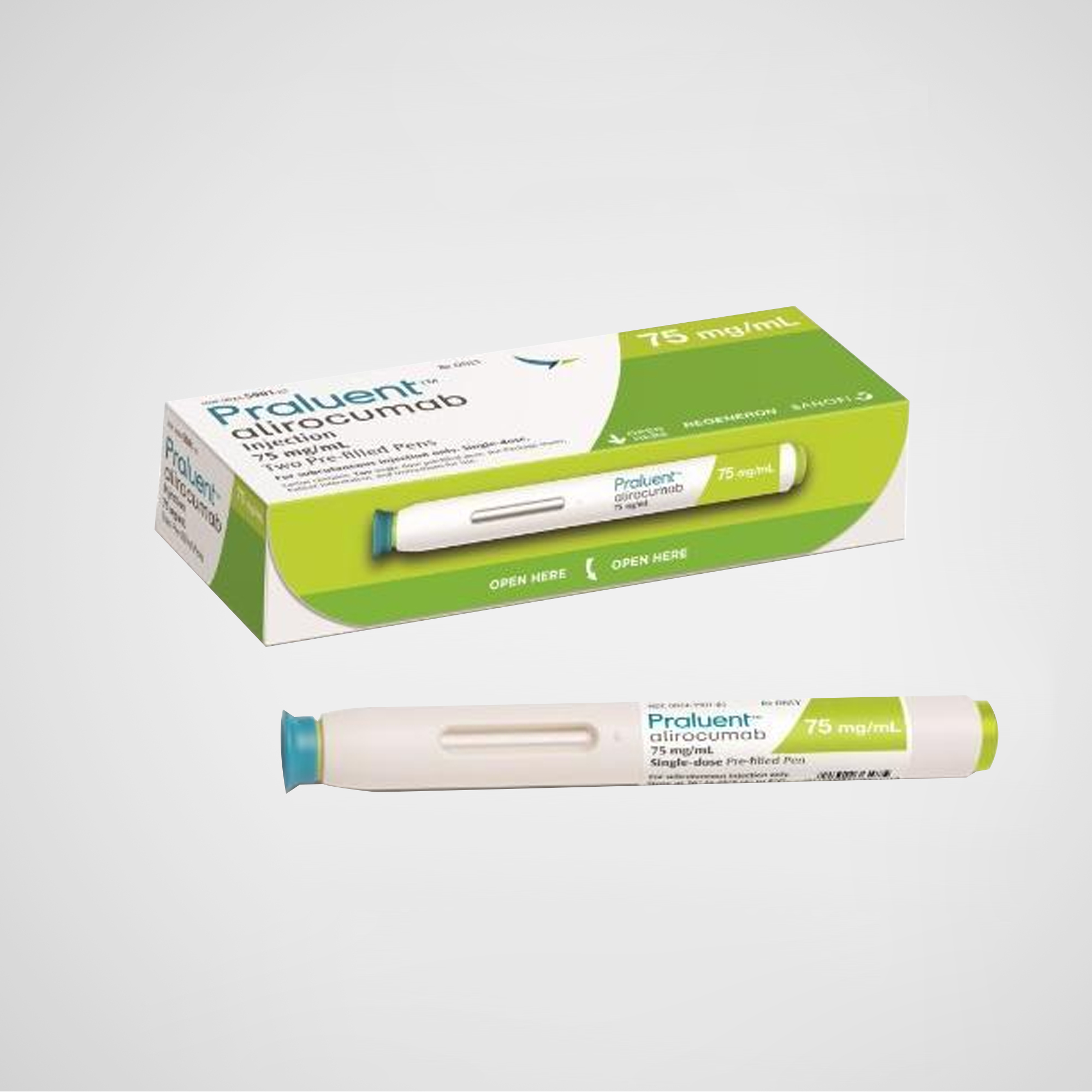
Praluent® – Alirocumab Injection
Praluent® – Alirocumab Injection | 75mg/mL or 150mg/mL | U.S.-Manufactured Global Supply
Praluent® (alirocumab) is a fully human monoclonal antibody that inhibits PCSK9, leading to significantly reduced LDL-C levels. Approved for use in patients with heterozygous familial hypercholesterolemia or clinical atherosclerotic cardiovascular disease, it is administered subcutaneously every 2–4 weeks. Manufactured under strict quality standards in the U.S., Praluent is supplied globally with original packaging for compliant export.
| Field Name | Example Value |
|---|---|
| Product Name | Praluent® – Alirocumab Injection |
| Product Code | PRLNT-US-ALIR-75/150 |
| Product Type | Monoclonal Antibody / PCSK9 Inhibitor |
| Category | Cardiovascular / Cholesterol Management / Specialty Biologics |
| Manufacturer | Sanofi–Regeneron |
| Appearance | Clear to slightly opalescent solution in pre-filled pen or syringe |
| Dosage / Units | 75mg/mL or 150mg/mL (1mL per pre-filled pen or syringe) |
| Purity | ≥ 99% recombinant human IgG1 monoclonal antibody (alirocumab) |
| Quantity Options | Box of 2 pre-filled pens or syringes |
| Storage | Store refrigerated at 2–8°C; may be kept at room temperature (≤25°C) for up to 30 days |
| Shelf Life | 24 months unopened |
| Packaging | Original U.S. or international packaging with serial number and multilingual insert |
| Manufacturer Origin | United States |
| Use Classification | Export Only – Prescription biologic; for use by licensed professionals and institutions only |
| Tags | praluent, alirocumab, PCSK9 inhibitor, monoclonal antibody, cholesterol injection, cardiovascular, BLUEBIOTECH |
⚠️This product is labeled for Research Use Only (RUO). Not for human or veterinary therapeutic use.
See RUO Declaration for more info.
Praluent® – Alirocumab Injection | 75mg/mL or 150mg/mL | U.S.-Manufactured Global Supply
Product Overview
Praluent® (Alirocumab) is a human monoclonal antibody targeting proprotein convertase subtilisin/kexin type 9 (PCSK9), used to lower LDL-C levels in patients with hypercholesterolemia, familial hypercholesterolemia (HeFH), or clinical atherosclerotic cardiovascular disease (ASCVD). Co-developed by Regeneron and Sanofi, Praluent is part of a new class of PCSK9 inhibitors proven to reduce cardiovascular risk when added to statin therapy.
This listing includes 75mg/mL and 150mg/mL pre-filled syringes or pens, suitable for subcutaneous self-injection every 2 weeks or monthly depending on dosage. Product is manufactured in the United States and sourced through the global pharmaceutical supply chain, with export-ready labeling.
📘 For detailed clinical trials, see PubMed – Alirocumab Studies
Key Features & Benefits
-
✅ PCSK9 Inhibition for LDL Reduction
Praluent prevents PCSK9 from binding LDL receptors, thereby enhancing LDL clearance from the bloodstream. -
✅ Proven Cardiovascular Risk Reduction
In the ODYSSEY OUTCOMES trial, Praluent significantly reduced the risk of MI, stroke, and unstable angina in high-risk patients. -
✅ Flexible Dosing Options
-
75mg/mL – Standard LDL-C control
-
150mg/mL – For patients requiring intensive lipid-lowering
-
-
✅ Patient-Friendly Delivery System
Offered in pre-filled pens or pre-filled syringes for at-home administration with minimal discomfort. -
✅ US-Manufactured, Global Label Variant
Identical formulation to U.S. domestic version; only label language differs to meet global regulatory needs.
Product Specifications
-
Product Name: Praluent® (Alirocumab)
-
Strengths Available: 75 mg/mL or 150 mg/mL
-
Dosage Form: Injection, subcutaneous
-
Device Type: Pre-filled syringe or autoinjector pen
-
Origin: United States
-
Packaging: 1 or 2 pens/syringes per carton (varies by region)
-
Storage: Refrigerated at 2°C – 8°C
-
Shelf Life: 18–24 months (depending on lot)
-
Use Frequency: Every 2 weeks or monthly (as per clinical protocol)
Recommended Indications
-
Adults with primary hyperlipidemia, including heterozygous familial hypercholesterolemia (HeFH)
-
Patients with clinical ASCVD who require additional LDL-C lowering beyond statins
-
Individuals intolerant to statins or unable to achieve lipid goals with oral therapy alone
How It Works
Alirocumab is a fully human IgG1 monoclonal antibody that binds to circulating PCSK9, preventing it from degrading LDL receptors on hepatocytes. This enhances the liver’s ability to remove LDL cholesterol, leading to dose-dependent reductions in LDL-C levels—often exceeding 50% when combined with statins.
📘 Learn more at FDA Label Information for Praluent
Compliance Statement
This product is manufactured in the United States and supplied through a global pharmaceutical distribution channel. Intended strictly for licensed healthcare professionals and institutional buyers. Product labeling and language may differ to meet destination country regulations. All importers and end users must comply with local laws governing monoclonal antibody therapeutics.
Tags / Keywords
Praluent Alirocumab Injection, PCSK9 Inhibitor Monoclonal Antibody, LDL Cholesterol Reducer, Praluent Global Supply, 75mg 150mg PCSK9, Sanofi Regeneron Praluent, Hyperlipidemia Injectable Treatment, US Export Biologic, Praluent PFS Pen
You might also like
Similar Products
Corporate Advantages

High-Purity Standards
Tested for purity and identity. COAs available
for all RUO compounds

Buyer Protection
Protected and discrete fulfillment
available upon request

Research-Grade Support
Dedicated service for labs, clinics, and
compounders handling RUO items.

Lab-Grade Quality Assurance
All compounds are tested for-identity and purity, COAs and supporting documentation are available upon request.

Strict RUO Compliance
All products are labeled for Researd Use Only (RUO), Not intended for human or veterinary therapeutic or diagnostic purposes.
Lab-Grade Quality Assurance

Custom branding.
white-label optionons and formulation assistancee available for eligible research institutions and partners.
Custom R&D & APl Synthesis

We support original
pharmaceutica solutions.custom research projects, and NDC-grade materials-meeting speifica.tions for qualified partners.

Serving Professionals Across the U.S.& Mexico
Trusted by MedSpas, wellness clinics and licensed compounders under licensed compounding relationships.
Secure & Discreet Fulfillment
Cold-chain shipping : private, plain.wrap packaging, and tracking for sensitive RUO materials.

⚠️ All compounds are provided strictly for Research Use Only (RUO). BLUEBIOTECH does not endorse or support clinical, therapeutic, or diagnostic use of its products unless under licensed compounder supervision.







![Mounjaro®-Tirzepatide injection [Japan Edition]](https://www.bluebiotech.health/wp-content/uploads/2024/11/Mounjaro-Tirzepatide-Made-in-Japan-7.5-600x800.jpg)

