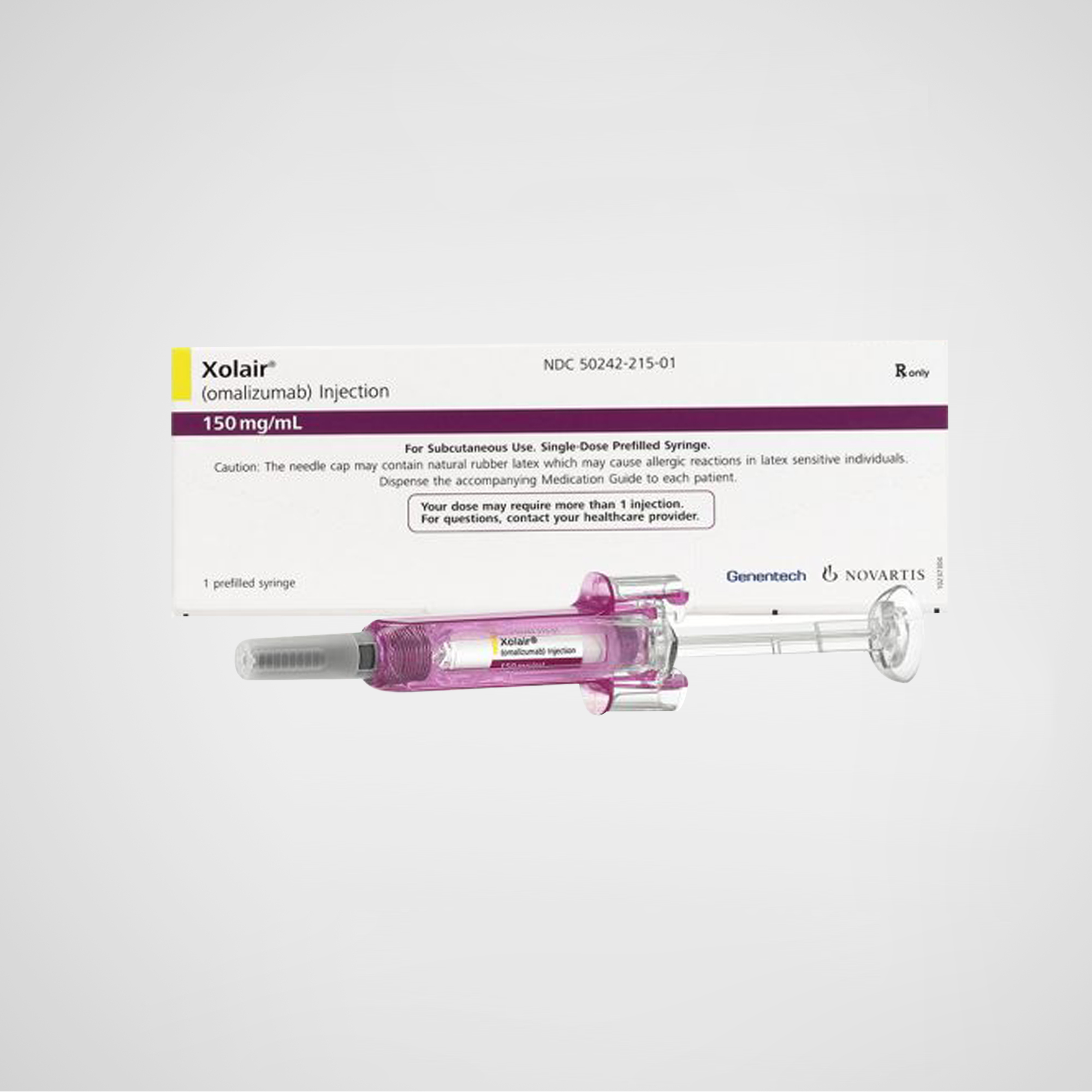
Xolair® – Omalizumab Injection
Xolair® (omalizumab) is a humanized monoclonal antibody that binds to immunoglobulin E (IgE), used in the treatment of moderate-to-severe allergic asthma and chronic spontaneous urticaria. Manufactured in the U.S. and supplied globally in export-compliant packaging.
| Field Name | Example Value |
|---|---|
| Product Name | Xolair® – Omalizumab Injection | U.S.-Manufactured Global Supply Monoclonal Antibody |
| Product Code | XOL-USA-150 |
| Product Type | Branded Biologic / Monoclonal Antibody |
| Category | Immunology / Allergy / Asthma |
| Manufacturer | Genentech, Inc. (A Member of the Roche Group) / Novartis Pharma AG (co-marketing) |
| Appearance | White to off-white lyophilized powder in single-use sterile vial |
| Dosage / Units | 150mg per vial (for reconstitution) |
| Purity | Pharmaceutical grade (originator biologic, sterile, preservative-free) |
| Quantity Options | N/A |
| Storage | Store refrigerated at 2–8°C; do not freeze; protect from light |
| Shelf Life | 24 months unopened |
| Packaging | Global packaging with multilingual insert; U.S. FDA and international export-compliant labeling |
| Manufacturer Origin | United States |
| Use Classification | Export Only – For institutional, research, or regulatory evaluation use outside U.S. domestic market |
| Tags | xolair, omalizumab, monoclonal antibody, IgE inhibitor, asthma, chronic urticaria, biologic injection, BLUEBIOTECH |
⚠️This product is labeled for Research Use Only (RUO). Not for human or veterinary therapeutic use.
See RUO Declaration for more info.
Xolair® – Omalizumab Injection | U.S.-Manufactured Global Supply Monoclonal Antibody
Product Overview
Xolair® (omalizumab) is a recombinant humanized monoclonal antibody (IgG1κ) developed by Genentech, Inc. (a member of the Roche Group) and Novartis Pharmaceuticals. It is the first anti-IgE monoclonal antibody approved globally for the treatment of moderate to severe allergic asthma, chronic spontaneous urticaria (CSU), and other IgE-mediated allergic conditions.
The Xolair® product offered here is manufactured in the United States by the originator company and supplied through the official global distribution chain. While packaging and labeling are adapted for destination country compliance (e.g., language, regulatory marks), the formulation, device, and manufacturing quality are 100% identical to the U.S.-market version.
Key Features & Advantages
-
✅ Original Brand, U.S.-Made by Genentech / Novartis
Not a biosimilar. Manufactured under strict FDA and cGMP compliance. -
✅ Biologically Identical to U.S.-Marketed Xolair®
Only packaging language may differ for international regulatory purposes. -
✅ Proven Mechanism Against IgE-Mediated Inflammation
Extensively studied and used in both respiratory and dermatological indications. -
✅ Multiple Indications Globally
Approved for moderate to severe allergic asthma (≥6 years), chronic spontaneous urticaria (≥12 years), and nasal polyps (in some regions). -
✅ Distributed via Global Supply Chain
Available to licensed importers, hospitals, and regulatory-approved partners worldwide.
Product Specifications
-
Product Name: Xolair® (omalizumab injection)
-
Active Ingredient: Omalizumab (recombinant humanized monoclonal antibody, IgG1κ)
-
Strength / Form: 75 mg or 150 mg per vial (lyophilized powder); single-use
-
Administration: Subcutaneous injection
-
Manufacturer: Genentech, Inc. (USA) / Novartis Pharmaceuticals
-
Packaging: International version with destination-specific labeling; identical formulation as U.S. version
-
Storage: 2°C–8°C refrigerated; protect from light
-
Shelf Life: 18–24 months depending on batch
-
Supply Category: Originator biologic; not a biosimilar or generic substitute
Mechanism of Action
Xolair® works by binding to free circulating IgE, thereby preventing its interaction with FcεRI receptors on mast cells and basophils. This reduces the allergic inflammatory cascade and helps stabilize immune response in conditions where IgE plays a central pathological role.
It is particularly effective in:
-
Reducing asthma exacerbation frequency
-
Improving control of chronic urticaria
-
Minimizing use of corticosteroids in long-term allergy management
More info: NIH Omalizumab Mechanism Overview
Use Cases in Global Supply
-
Licensed importers supplying hospitals and allergy clinics
-
Cross-border institutional procurement under legal frameworks
-
Research hospitals conducting original biologic comparison studies
-
Academic centers studying IgE-mediated disorders
Compliance Statement
This product is manufactured in the United States by Genentech and Novartis, and is part of the official global supply chain. The formulation, quality, and pharmacological profile are identical to the U.S.-market version. Packaging and labeling are customized for compliance with local regulatory requirements in the export destination. This product is not a biosimilar. All importers and users must ensure compliance with local laws and licensing requirements.
Tags / Keywords
Xolair Global Supply, Omalizumab Original Biologic, Anti-IgE Antibody, Chronic Urticaria Treatment, Severe Allergic Asthma Therapy, U.S.-Manufactured Xolair, Monoclonal Antibody for Allergy, Global Distribution Biologic, Genentech Novartis MAb, FcεRI Blocker
You might also like
Similar Products
Corporate Advantages

High-Purity Standards
Tested for purity and identity. COAs available
for all RUO compounds

Buyer Protection
Protected and discrete fulfillment
available upon request

Research-Grade Support
Dedicated service for labs, clinics, and
compounders handling RUO items.

Lab-Grade Quality Assurance
All compounds are tested for-identity and purity, COAs and supporting documentation are available upon request.

Strict RUO Compliance
All products are labeled for Researd Use Only (RUO), Not intended for human or veterinary therapeutic or diagnostic purposes.
Lab-Grade Quality Assurance

Custom branding.
white-label optionons and formulation assistancee available for eligible research institutions and partners.
Custom R&D & APl Synthesis

We support original
pharmaceutica solutions.custom research projects, and NDC-grade materials-meeting speifica.tions for qualified partners.

Serving Professionals Across the U.S.& Mexico
Trusted by MedSpas, wellness clinics and licensed compounders under licensed compounding relationships.
Secure & Discreet Fulfillment
Cold-chain shipping : private, plain.wrap packaging, and tracking for sensitive RUO materials.

⚠️ All compounds are provided strictly for Research Use Only (RUO). BLUEBIOTECH does not endorse or support clinical, therapeutic, or diagnostic use of its products unless under licensed compounder supervision.

![Liztox® – Botulinum Toxin Type A [South Korea]](https://www.bluebiotech.health/wp-content/uploads/2024/12/Liztox-Type-A-100ui-1-600x800.jpg)


